-
Scanning tunneling microscopy image contrast of monolayer platinum on graphite
F. Atamny, T. Bürgi, R. Schlögl and A. Baiker
Surface Science, 475 (1-3) (2001), p140-148


DOI:10.1016/S0039-6028%2800%2901098-0 | unige:14757 | Article HTML | Article PDF
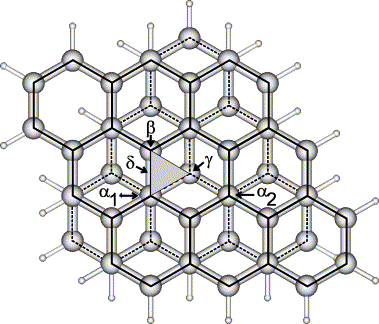
Platinum particles supported on graphite have been investigated by scanning tunneling microscopy (STM). For one monolayer thick Pt particles the individual Pt atoms form a characteristic intensity pattern due to a mismatch between the Pt and graphite lattice. Based on density functional theory calculations and model structures of Pt on graphite it is argued that the observed STM imaging contrast has its origin in the tip induced elastic deformation of the graphite underneath the Pt particle. The Ptâgraphite potential is much stiffer than the graphiteâgraphite potential. The calculations furthermore indicate that Pt adsorption is favored over top rather than hole sites and that the barrier for diffusion is very low.